The first FDA-approved PCAB
VOQUEZNA® (vonoprazan) is a potassium-competitive acid blocker (PCAB) that may selectively concentrate in the parietal cells in both the resting and stimulated states, suppressing acid by inhibiting the binding of potassium ions to the acid pump.1
PCAB
PPI
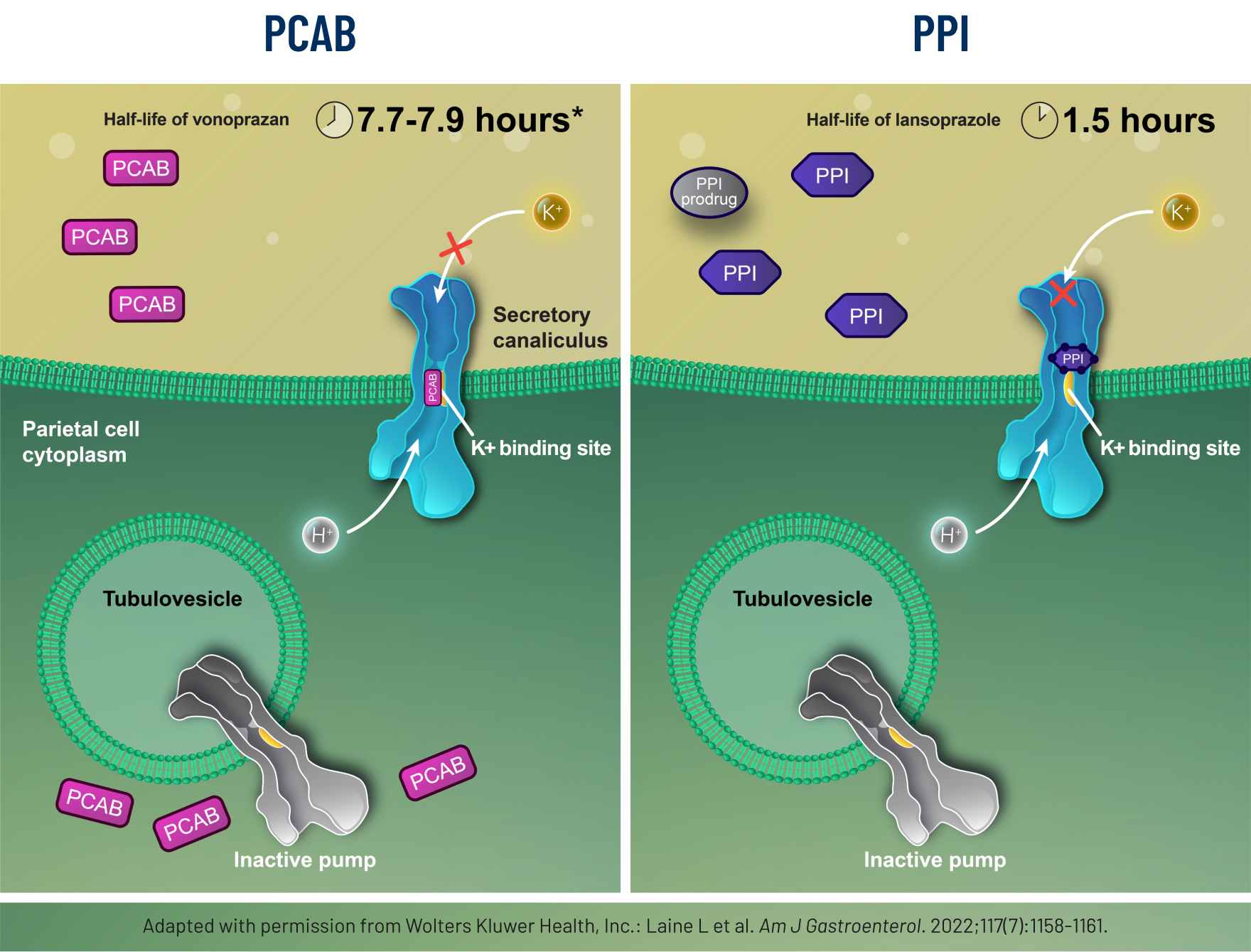
Features of vonoprazan include1:
- Acid is not required for activation
- Binds to active acid pumps in a noncovalent and reversible manner
- Long half-life (7.7-7.9 hours) *
