The first FDA-approved PCAB
Adapted with permission from Wolters Kluwer Health, Inc.:
Laine L et al. Am J Gastroenterol.
2022;117(7):1158-1161.
2022;117(7):1158-1161.
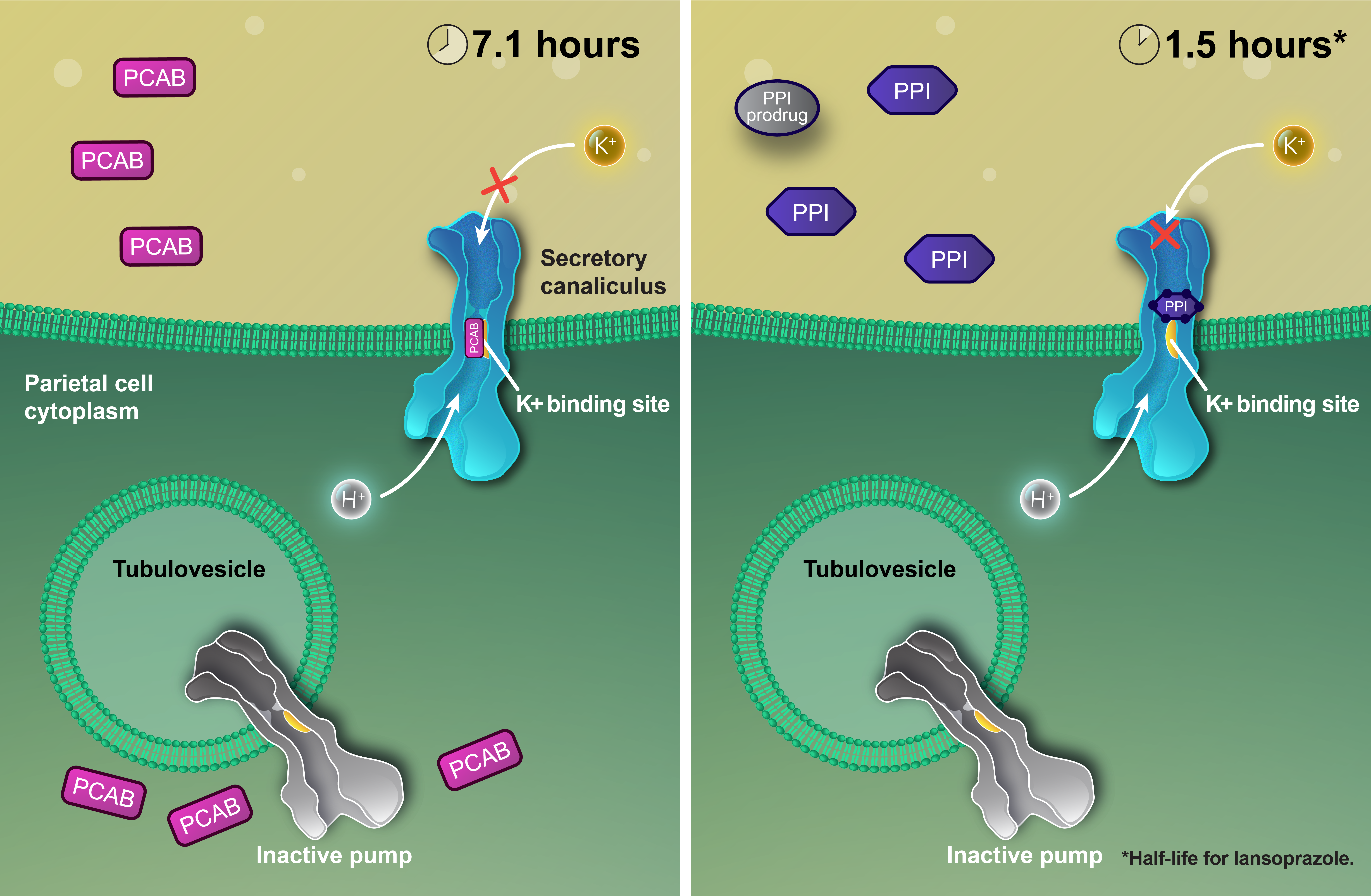
The clinical significance of these mechanistic differences has not been established.
VOQUEZNA® (vonoprazan) is a potassium-competitive acid blocker (PCAB) that1:
- May selectively concentrate in the parietal cells in both the resting and stimulated states
- Suppresses acid by inhibiting the binding of potassium ions to the acid pump
Features of vonoprazan include1:
- Acid not required for activation
- Binds to active acid pumps in a noncovalent and reversible manner
- Long half-life (7.1 hours)